Chapter 6 - Data Sets, $DATA and $INPUT Records, and the Data Preprocessor
This chapter tells how to create data for analysis by NONMEM-PREDPP. It tells how to describe the data using $DATA and $INPUT records. The requirements for formatting the data for NONMEM-PREDPP are somewhat more stringent than are the requirements for formatting the data for NM-TRAN. The Data Preprocessor is a component of NM-TRAN which modifies the data so that it becomes formatted appropriately for NONMEM-PREDPP.
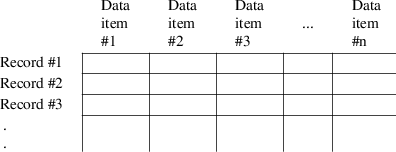
A data set for NONMEM analysis consists of a series of records ("lines" in the terminology of editing programs). Each record must consist of a fixed number of data items and each must have the same format. Figure 6.1 shows how such a data set may be pictured. In data base terminology, this is a "flat" structure.

Figure 6.1. A NONMEM input data set. Each data record is a row; each type of data item is in a different column.
NONMEM imposes no limit on the number of records in the data set. It does not (nor does PREDPP or NM-TRAN) sort the data records before processing them, so the data records must already be in the correct sequence.
NM-TRAN may be instructed to omit records. See the DROP and IGNORE options of the $DATA record, below,
With NONMEM 7.5, PRED may also instruct NONEMEM to omit records. See PRED_IGNORE_DATA in Chapter 12, Section 6.1.
NONMEM reads records from the
data set with a FORTRAN FORMAT specification, and so each
data item must occupy a fixed number of character positions.
Data items are always numbers. However, if no particular
number is appropriate for a given data item on a given
record, the data item is called a null data item; it
may be given the numerical value 0 or the nonnumerical value
".", or left blank. Zero’s were used in the
first two lines of the Theophylline example of Chapter 2,
which appeared as follows:
2 320. 0. 0.
2 0. .27 1.71
The Data Preprocessor allows each value in the data set to
occupy only as many character positions as it needs, so long
as the data items are separated by blanks (spaces) or
commas. Tab characters may also be used as separators if
they are stored as explicit characters, e.g., ASCII 011,
although this is platform-dependent and should be tested
carefully. When there are no commas or tabs, the value
"." or 0 must be used to hold the place of
a null data item. The two lines above could have been
entered as follows:
2 320. 0. 0.
2,,.27,1.71
(Note the use in the second line of adjacent commas
",," to denote a null data item.)
The contents of the data items must be purely numeric; i.e., character values such as Y, N, M or F may not be recorded. Instead, numeric codes such as 0 or 1 must be used.
With NONMEM VI, the number of data items per data record is given by constant PD in file SIZES. The default value is 20. With NONMEM 7.1, the default value is 50. With NONMEM 7.2, there is no limit on the number of data items per data record. If the value in SIZES is not sufficient, a larger value may be specified on the $SIZES record.
Clinical data often has a "hierarchical" file structure, with (say) two record formats: a patient record, containing fixed information about a patient (ID number, sex, age, prior history of smoking or drug use, etc), followed by one or more visit records, containing doses and physical observations during the course of the study. Visit records may not even contain the same number of items as patient records, nor have the same format. The hierarchy is shown schematically in figure 6.2.
Patient record
Visit record
Visit record
...
Patient record
Visit record
Visit record
...
Figure 6.2. A hierarchical data file. Patient and visit records have different formats.
NONMEM cannot accept such data. For NONMEM, the (fixed) information on the "patient" record must be copied onto every "visit" record, and the "patient" records must be eliminated. This is the user’s responsibility and is typically done in a one-time data conversion step using the system editor and/or a specially written computer program. If an individual’s data is to be deleted because he did not complete the study or had an adverse outcome, it should be done at this time. In addition, numeric codes should be substituted for alphabetic codes. Clinical data sometimes includes multi-digit, non-consecutive patient identification numbers drawn from some patient identification system. Such patient identification numbers can be used with NONMEM as the identification data item described in Section 6.2. However, it is preferable to append to each patient’s data records numbers from the sequence 1, 2, 3, ..., for use as the ID data item. This will make it easier to read a scatterplot which includes ID along one of the axes (e.g., residual vs ID).
When there is a large amount of data, we strongly suggest that a small amount of data (from one or two individuals) be prepared for NONMEM-PREDPP analysis and a run in which only tables and scatterplots are output be made to check that the data is correctly prepared before a great deal of labor is expended on the remainder.
When PREDPP is used with NONMEM, the data must meet additional requirements. First, PREDPP is concerned with time-ordered events such as dose events, which introduce drug into the system at particular times, and observation events, which report observations taken at particular times. PREDPP insists that these events be recorded on separate records. That is, dosing information cannot be recorded on the same record as an observed value. Second, PREDPP requires that the time of each event be recorded on each data record, and that the physical sequence of the data records be the same as their sequence in time. (E.g., if a dose event immediately precedes an observation event in time, then the dose event record must immediately precede the observation event record.) Again, neither PREDPP nor the Data Preprocessor will physically sort or resequence the data records.
The $DATA record describes the characteristics of the external data file to be processed by NONMEM. NONMEM is not a data base management system and does not store a data set between runs; once a file has been prepared for NONMEM, it must be re-read each time it is to be analyzed. The first character string appearing after $DATA is the name of the file containing the data. Since it is to be used in a FORTRAN OPEN statement, this name may not include embedded special characters such as slashes (/ or \), commas, semi-colons, parentheses, equal signs or spaces unless it is surrounded by single quotes ’ or double quotes ". The filename may contain 80 characters. (If a file is to be opened by NONMEM rather than by NM-TRAN, the filename may not contain embedded spaces, and may contain at most 71 characters.) A FORTRAN format specification suitable to read the data may follow the file name; this is optional and can be supplied by the Data Preprocessor. The choice is discussed more fully in Section 10.4 of this chapter.
Certain options may be specified if desired. Among these are:
|
RECORDS=n |
This tells the number of records to be read from the data file. If omitted, the records are read to the end-of-file or to a NONMEM FINISH record (Users Guide II). The RECORDS option may be used to limit NONMEM processing to the initial portion of the file and is useful during the early stages of debugging.
|
RECORDS=label |
"Label" is a data item label. The data records for the problem will start at the place where the file is positioned before data records are read and include all contiguous data records having the same value for the data item. In particular, the ID label may be used (or alternatively, the option may be coded RECORDS=IR, RECORDS=INDREC, or RECORDS=INDIVIDUALRECORD) to obtain the data for a single individual.
|
IR,INDREC,INDIVIDUALRECORD. |
|
|
NOREWIND|REWIND |
With the first problem
specification in a control stream, the file is positioned at
its initial point so that the first record in the file is
used. The options REWIND and NOREWIND apply only with a
$DATA record in a subsequent problem specification.
REWIND: Reposition the file at the start.
NOREWIND: Leave the file at its current position so that the
next record in the file is read. Used when the $DATA record
with the previous problem specification included the RECORDS
option so that NM-TRAN did not read to a physical
end-of-file. This is the default.
|
LRECL=n |
This tells the length of the physical data records. It is required if the operating system associates a fixed physical record length with every disk file and considers it a fatal I/O error if a READ command requests more characters than the records contain. If this is true of your operating system, the operating system will issue an error message when you first run NM-TRAN without the LRECL option in the $DATA record.
|
WIDE |
This requests that the NONMEM data set produced by NM-TRAN always contain single-line records, and that these records always include at least one space between data items. Such a data set can be further processed by other programs. (The default is NOWIDE, in which case NM-TRAN limits the records to 80 characters by creating multi-line records and/or eliminating spaces between data items if necessary.) It may not be used if a FORTRAN format specification is present. It also provides an extra character for relative times computed by the Data Preprocessor. |
NULL=c
|
This requests that the NONMEM data set produced by NM-TRAN contain the character c in place of null data items. For example, NULL=0 requests that all null data items be replaced by 0. The syntax NULL=’c’ and NULL="c" is also permitted. The default is NULL=’ ’. It may not be used if a FORTRAN format specification is present. |
|
IGNORE=c |
This instructs NM-TRAN to
ignore data records having character c in the first
character position ("column 1") of the record.
This allows the use of "comment" records in the
NM-TRAN data set. The syntax IGNORE=’c’ and
IGNORE="c" is also permitted. It may be used even
if a FORTRAN format specification is present.
The character @ has a special meaning. It signifies that any
data record containing an alphabetic character (or special
characters @ or #) as its first non-blank character (not
just in column 1) should be ignored. Alphabetic characters
are the letters A-Z and a-z. Thus, a table file produced by
NONMEM in an earlier run can be used as an NM-TRAN data set.
Any header lines included in this table can be dropped by
specifying IGNORE=@.
When the IGNORE option is omitted, any records containing the character # in column 1 are ignored.
|
IGNORE=(list), ACCEPT=(list) |
This form of the IGNORE option
allows records to be dropped based on the values of data
items. For example,
IGNORE=(GEN.EQ.1,AGE.GT.60).
Records having GEN equal to 1 or AGE greater than 60 are
dropped. All others are accepted. The ACCEPT option allows
records to be accepted based on the values of data items.
FORTRAN logical operators .EQ., .NE., .GT., .GE., .LT., .LE.
may be used, as well as FORTRAN 90 logical operators ==, /=,
>, >=, <, <=.
Special operators .NEN. and .EQN. request that character
strings be converted to numeric prior to being compared
(nm73). See Guide VIII for more information.
|
LAST20=nn |
"nn" is a 2 digit number that specifies the highest 2-digit year that is assumed to be in the 21st. century, i.e., that represents 20nn rather than 19nn. See Section 10.1.5 below.
|
TRANSLATE |
The translate option must be followed by parentheses enclosing a list of one or more translate specifications. For example,
$DATA filename TRANSLATE(TIME/24,II/24)
Translate specification TIME/24 causes the value of TIME to be divided by 24, whether or not day-time translation occurs (i.e., whether or not relative times are being computed). This has the effect of changing the unit of TIME from hours to days. Similarly, translate specification II/24 causes the value of II (interdose interval) to be divided by 24 whether or not ":" appears in any II value. With NONMEM 7.3, any value may be given for dividing time and II values, and any precision may be requested. See Section 10.1.4 below.
This record describes how many data items there are on each data record, the order of the data items, and tells what the labels of the data items are.
A data item label is one to four
letters (A-Z) or numerals (0-9). With NONMEM 7 a label
consists of 1-24 letters (A-Z), numerals (0-9), and the
character ’_’. (The length 24 is specified by
constant SD in SIZES)
The first character must be a letter. These labels are the
ones which will be used in other records (such as $PK or
$SCATTERPLOT), and will appear in NONMEM’s output. The
order of the data items on the data records is not
important, but must be the same on all data records in the
data set. In both the examples of Chapter 2, the ID data
items happened to be the first ones in the data records, and
the DV data items happened to be the last ones. This order
was arbitrary.
Certain data item labels are reserved in that they identify data items which are recognized specifically by NONMEM, PREDPP, or NM-TRAN. The data items they label are themselves called NONMEM, PREDPP, or NM-TRAN data items, respectively.
|
• |
Reserved NONMEM data item labels are: ID, L2, DV, and MDV. They are discussed in Section 6 of this chapter and in Section 4.2 of Chapter 12. Additional reserved NONMEM data item labels are: MRG_, RAW_, and RPT_. See Guide VIII for a discussion of these items. | |
|
• |
Reserved PREDPP data item labels are: TIME, EVID, AMT, RATE, SS, II, ADDL, CMT, PCMT, CALL, and CONT. They are discussed in Section 7 of this chapter and in Section 2.4 of Chapter 12. With NONMEM 7.2, additional reserved PREDPP data items are the extra EVID labels, XVID1, XVID2, XVID3, XVID4, and XVID5. See Guide VIII for a discussion of these items. | |
|
• |
Reserved NM-TRAN data item labels are: DATE, DAT1, DAT2, DAT3, and L1. DATE, DAT1, DAT2, and DAT3 are discussed in Section 10.1 of this chapter; L1 is discussed in Section 4.2 of Chapter 12. |
If you do not want to use the
reserved label, you can supply two labels: the reserved
label and a "synonym". Either label can be used in
subsequent records, but only the synonym will appear in
NONMEM output. For example,
$INPUT PNO=ID,CONC=DV,DOSE=AMT,WT,....
The first three data items are given the labels PNO, CONC,
and DOSE. These labels are synonyms for the NONMEM data
items ID and DV and for the PREDPP data item AMT. The last
data item is given the label WT and is not a reserved data
item; it is an example of fixed effect
("concomitant") data

When $PK and $ERROR records are present, certain labels may not be used at all as data item labels. These are: the labels for the basic and additional PK parameters for the pharmacokinetic model, as listed in Appendices 1 and 2 (e.g., for ADVAN1 and TRANS2: CL, V, S1, S2, F1, F0), and specific labels for the arguments of the PK and ERROR subroutines: IDEF, IREV, N, GG, IRGG, HH, and G.
If no format specification is included on the $INPUT record, then another synonym, DROP, may be used with any data item. DROP may be used as a synonym more than once. It identifies data items to be dropped (i.e. eliminated) from the NM-TRAN data set by the Data Preprocessor while constructing the NONMEM data set. This provides a way to limit the number of data items in the NONMEM data set and to eliminate non-numeric data items.
There must always be a Dependent Variable data item labeled DV. This is a value of an observation. There can be only one DV data item per data record. The position of the DV data item (and the ones described below) is not important. However, its position must be the same on all records.
When the data is from a population, NONMEM expects the Identification data item, labeled ID, and expects the data to be organized into two or more "individual records". An individual record is a group of contiguous data records having the same value for the ID data item and presumably containing data from the same individual. ID data item values need not be consecutive, increasing, unique, nor begin with 1. E.g., 3, 5, 6, 10, 3, etc. is a possible sequence of ID values. Note the two instances of 3 as ID data item values. As long as these two instances are separated by different ID data item values (e.g. 5, 6, 10), they represent different individuals.
If there are records in an input data set which do not contain values of observations, then NONMEM needs to be informed of this fact. This is done using the Missing Dependent Variable data item labeled MDV. The values of MDV are:
|
0 |
The DV data item of the data record contains a value of an observation. The record is referred to as an observation record. | |
|
1 |
The DV data item of the data record does not contain a value of an observation. |
NONMEM 7 limits the number of observation records per individual record to 250. To change this limit, see Users Guide III. With NONMEM 7.3, there is no limit on the number of observation records.
When PREDPP is used, the Data Preprocessor is able to recognize which records contain observed values and which do not, and it can supply the MDV data item if it is not already present in the data set, i.e. if the label MDV does not occur in the $INPUT record. (When PREDPP is not used, the Data Preprocessor cannot do this.)
PREDPP will in general need the Time data item, labeled TIME. With NONMEM 7.4, the value of TIME may be negative. With earlier versions of NONMEM, the value of TIME must be non-negative. Within an individual record, values of TIME may not decrease. (Exceptions exist for reset and reset-dose events; see Section 7.3.) The units are optional (e.g., minutes or hours), but should be consistent with other units used in the problem. The TIME of the first event record may be zero or non-zero. (If non-zero, then PREDPP in effect subtracts this value from all other TIME values within the same individual record, so that PREDPP always works with relative time values.) The Data Preprocessor permits TIME to be expressed as clock time (e.g., 8:30, representing the time, half-past 8 o’clock). Such times are converted by the Data Preprocessor into relative times. Details are given in Section 10.1 below.
Doses are described using one or more of these four data items, depending on the kind of dose. A detailed discussion of these data items and of dose records in general is deferred to Section 8 below.
When PREDPP is used, all data records are also called event records. Every event record must contain an Event Identification data item identifying the kind of event described by the record, and labeled EVID. The values of EVID and the five kinds of event records are:
|
0 |
Observation event. This record contains an observed value (in the DV data item). Dose-related data items such as RATE and AMT must be 0. | |
|
1 |
Dose event. This record describes a dose. The contents of the DV data item are ignored. | |
|
2 |
Other event. This record is used for a variety of purposes. It can be used to obtain a predicted value at a point in time at which no actual observation or dose event took place; it can be used to turn a compartment off or on at a point in time; it can be used to mark a time at which a change in a physiological data item (e.g. weight) occurs (as well as give the new value of the data item). Dose-related data items must be 0. The contents of the DV data item are ignored. | |
|
3 |
Reset event. This record is used to reset the kinetic system at some point in time, without actually starting a new individual record: time is set to whatever time appears in the event record, the amounts in each compartment are set to zero, prior doses are cancelled, and the on/off status of each compartment is set to its initial status. It is in all other respects identical to an other event type record. It is typically used within an individual record, when the individual had a course of drug treatment, followed by a wash-out period, followed by another course of drug treatment. It should appear prior to the start of the second course. | |
|
4 |
Reset-dose event. This record combines EVID types 3 (reset) and 1 (dose). First the system is reset, and then a dose is introduced. It is in all other respects identical to an ordinary dose event type record. |
If only dose and observation event records are present in the NM-TRAN data set, and if EVID is not already present in the data set (i.e. EVID does not appear in the INPUT record), then EVID will be supplied automatically by the Data Preprocessor. This is what was done in the examples of Chapter 2. If other or reset type event records are present in the data set, then the $INPUT record must include the EVID data item, and the data set must include the appropriate values for EVID on all the data records.
The Compartment data item (CMT) and Prediction Compartment data item (PCMT) are similar. Both contain the number of a compartment in the model. (Compartments and compartment numbers are discussed in Chapter 7 and Appendix 1, as are default compartments. It may help to look at Chapter 7 and Appendix 1 at this time.) If CMT or PCMT is not defined in the data set (i.e., not listed in the $INPUT record), or has the value 0 on a given event record, the appropriate default compartment is used, except as noted below. This is what was done in the examples of Chapter 2. The meaning of the two data items depends on the particular kind of event record.
|
• |
Observation event: CMT specifies the compartment from which the predicted value of the observation is obtained. PCMT is ignored. When CMT specifies the output compartment, it is allowed to have a negative sign (e.g., with the One-compartment model, CMT may be -2). This signals that after the prediction is computed the output compartment is to be turned off, i.e. the amount in the compartment is to be set to zero. The amount remains zero until the compartment is subsequently turned on. This is quite useful with urine observations; see Section 9 below.† |
----------
†
This is also permitted with output-type compartments; see
Chapter 12, Section 2.8.
----------
|
• |
Dose event: CMT specifies the compartment into which the dose is introduced. The compartment is turned on if it was previously off. PCMT specifies the compartment for which a predicted observation is computed. This predicted value is not associated with an observation, but it can be useful because it will appear in tables or scatterplots. | |
|
• |
Other event: A positive value of CMT specifies that the compartment is to be turned on if it is off. A negative value of CMT specifies that the compartment is to be turned off if it is on. (If CMT is 0, no compartment is turned on or off.) PCMT is the same as for dose events. | |
|
• |
Reset event: CMT is ignored. PCMT is the same as for dose events. | |
|
• |
Reset-dose event: CMT and PCMT are the same as for dose events. |
The Call data item (CALL) is used to force a call to either or both of the PK and ERROR subroutines with the event record when such a call would not normally occur. A call to the PK or ERROR subroutine causes the code specified by the $PK or $ERROR records, respectively, to be executed with the event record. This is discussed in Chapters 7 ($PK) and 8 ($ERROR).) When not defined in the data set, CALL is assumed to be 0 always. The values are:
|
0 |
No forced call; PREDPP takes its normal action. | |
|
1 |
Force a call to ERROR. | |
|
2 |
Force a call to PK. | |
|
3 |
Force a call to both PK and ERROR. | |
|
10 |
Force a call to ADVAN9. May be combined with other values. E.g., the value 12 means "Force a call to PK and to ADVAN9". |
Doses are described using one or more of the data items discussed below. A detailed discussion of the actual kinds of doses that PREDPP recognizes follows in Section 8.2, including a precise definition of what is meant by the term "steady-state dose" (Section 8.2.3). A data item that is not needed to describe the kinds of doses used in the study need not be defined in the data set; it will in effect always have the value 0. Only AMT (Dose amount) was used in the examples of Chapter 2, for example. The values of dose-related data items should be 0 for non-dose events and for those dose events to which they are not relevant.
AMT data item
The Amount data item (AMT) gives the amount of a bolus dose or of an infusion of finite duration. This amount should be a positive number.
RATE data item
The Rate data item (RATE) gives the rate of an infusion. This rate should be a positive number. (Negative values are discussed in Chapter 12, Section 2.3.)
SS data item
The Steady-state data item (SS) can take four values.
|
0 |
This record does not describe a steady-state dose. | |
|
1 |
This record describes a steady-state dose. If this is not the first event record for the individual, then the system is first reset as if by a reset event record (except that the on/off status of the compartments is unchanged from what it was prior to the event record and the time on the event record must not be less than the time on the previous event record). The compartment amounts are then computed using steady-state kinetic formulas. | |
|
2 |
This record describes a steady-state dose. No reset of the kinetic system occurs. Compartment amounts are computed using steady-state kinetic formulas and are then added to the amounts already present at the event time. The use of SS=2 will be discussed further in Section 8.2.7, below. | |
|
3 |
This record describes a steady-state dose. It is exactly like a steady-state dose with SS data item = 1, except that existing compartment amounts and derivatives are retained and used as initial estimates. The computed steady-state levels replace these compartment amounts and derivatives. This value of SS may be specified only with SS6 and SS9 (the General Nonlinear Models). |
II data item
The Interdose Interval data item labeled II gives the time between implied doses (see Section 8.2.3 and Chapter 12, Section 2.4). For a steady-state infusion, it should be 0. For other steady-state doses, it should be a positive number whose units are the same as the TIME data item.
Any of the doses described here may be introduced into any compartment of the model except the output compartment. Examples are given below that are fragments of data records, identifying the data items of interest and showing their contents on the dose record. The units of various data items are presumed to be appropriate for some actual data.
All the examples in Chapter 2
involve instantaneous bolus doses, which we shall refer to
simply as bolus doses. (There is also such a thing as a
"zero-order bolus dose", see Chapter 12, Section
2.3.) These are dose records having AMT>0, RATE=0 and
SS=0. (Recall that if RATE and SS are not defined on the
$INPUT record, they are effectively 0.) If the $PK record
computes a bioavailability fraction parameter for the
compartment into which the dose is introduced, then the
contents of the AMT data item is multiplied by the current
value of this parameter before the amount is added to the
compartment. A bolus dose enters the dose compartment
immediately; the predicted (scaled) amount in the dose
compartment, if displayed in a table or scatterplot, will
include the dose.
Example:
TIME AMT
4. 10.
This is a dose of 10 to be added to the default dose
compartment at time 4.
A bolus dose to the central compartment might be interpreted as an IV bolus dose; to the depot it might be an oral tablet; to a peripheral compartment it might be an intra-muscular injection.
Infusions are doses having
AMT>0 and RATE>0. The duration of the infusion is
computed by PREDPP by dividing the AMT by the RATE. As with
bolus doses, AMT is first multiplied by the bioavailability
parameter for the dose compartment, if any. There is no
explicit "end of infusion" record. Drug amounts in
the system cannot be affected in a detectable way at the
time an infusion begins by any drug introduced by the
infusion; the predicted (scaled) amount in the dose
compartment, if displayed in a table or scatterplot, will
not include the dose. Infusions may overlap. That is,
subsequent dose records may start new infusions before old
ones have finished. It is not an error if an
infusion’s duration is so large as to extend beyond
the time of the last event record for the individual; the
remainder of the drug is ignored. A reset or reset-dose
event, or a steady-state dose event with SS=1, will also
terminate any infusions in progress.
Example:
TIME AMT RATE
4. 10. 2.
The duration of the infusion will be computed as 10./2., and
so the infusion, which begins at time 4, will terminate at
time 9. (=4.+5.).
An infusion to the central compartment might be interpreted as an IV infusion; to the depot it might be a sustained release tablet; to a peripheral compartment it might be an implant or skin patch which releases drug at a known constant rate. It is possible for NONMEM-PREDPP to estimate the input rate of a constant-rate drug delivery system (see Chapter 12, Section 2.3).
A steady-state dose can be
regarded as the last one of a series of doses just like the
one specified in the dose event record, which have been
given at a regular interdose interval since time
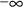 , and such that they have led to a steady-state periodic
pattern of drug amounts in the system by the time this last
dose has been administered. The doses of similar kind that
precede it are called implied doses, because their
existence is not described by separate dose records in the
data set, but rather is implied by the description of the
single steady-state dose. By stipulating that a dose is a
steady-state dose, the user instructs PREDPP to update the
drug amounts in the system at the time the dose is given by
using steady-state kinetic formulas. This can take less
computational time than using separate dose records to
describe the implied doses and using transient kinetic
formulas to advance the system from one dose time to the
next (as well as requiring fewer dose records). The formulas
used to compute the steady-state amounts at the time the
steady-state dose is introduced use the values of the basic
and additional pharmacokinetic parameters in effect at this
time; any values in effect at earlier times are ignored.
Moreover, when using a steady-state dose, the user is
assuming that under reasonable values of the pharmacokinetic
parameters, steady-state is in fact effectively reached by
the time the dose is introduced; PREDPP does not check this
assumption. The output compartment must be off when a steady
state dose record is encountered in the data set.
, and such that they have led to a steady-state periodic
pattern of drug amounts in the system by the time this last
dose has been administered. The doses of similar kind that
precede it are called implied doses, because their
existence is not described by separate dose records in the
data set, but rather is implied by the description of the
single steady-state dose. By stipulating that a dose is a
steady-state dose, the user instructs PREDPP to update the
drug amounts in the system at the time the dose is given by
using steady-state kinetic formulas. This can take less
computational time than using separate dose records to
describe the implied doses and using transient kinetic
formulas to advance the system from one dose time to the
next (as well as requiring fewer dose records). The formulas
used to compute the steady-state amounts at the time the
steady-state dose is introduced use the values of the basic
and additional pharmacokinetic parameters in effect at this
time; any values in effect at earlier times are ignored.
Moreover, when using a steady-state dose, the user is
assuming that under reasonable values of the pharmacokinetic
parameters, steady-state is in fact effectively reached by
the time the dose is introduced; PREDPP does not check this
assumption. The output compartment must be off when a steady
state dose record is encountered in the data set.
(The Model Event Time (MTIME) feature described in Chapter 12 does not apply during steady-state computations. The Absorption lag (ALAG) feature described in Chapter 12 does apply. See Guide VI, Chapter V, Notes 3 and 4.)
These are dose events having
AMT>0, RATE=0, SS=1, and II>0. The II data item
(interdose interval) tells how many time units apart the
doses were given. As with non-steady-state bolus doses, AMT
is first multiplied by the bioavailability parameter for the
dose compartment, if any.
Figure 6.3 shows how drug levels vary with time. The
concentration-time profiles over each interdose interval
look the same since, in principle, there is an
infinite number of implied doses.
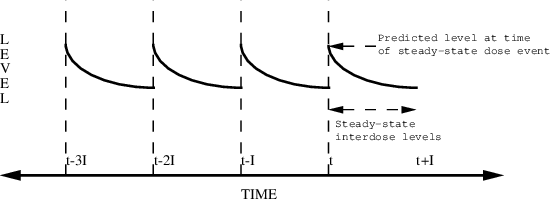
Figure 6.3. Steady-state with multiple bolus doses. The dose is given at time t. The interdose interval is I. Steady-state levels can be predicted between times t and t+I.
Example:
TIME AMT SS II
8 10. 1 12
Here, an infinite number of bolus doses, 10 units each, are
assumed to have been given 12 hours apart, with the last of
these given at time 8AM, at which time steady-state is
reached. The fact that TIME is 8 has no effect on the
computed amounts, but is important in relation to the
records that follow. Steady-state levels can be predicted at
any time between the time on the dose record (8) and the end
of the succeeding interdose interval (12) (provided there
are no further doses introduced during this
interval). If another (steady-state or
non-steady-state) dose just like the steady-state one
is introduced at time 20, then predictions in the interdose
interval following this time will also be steady-state
levels.
These are dose events having
AMT>0, RATE>0, SS=1, and II>0. Each such event
describes the last of a series of regularly spaced
infusions, all of the same amount and rate. As with a
non-steady-state infusion, the duration of each infusion is
given by AMT/RATE. The bioavailability fraction applies to
each infusion of the series.
Figure 6.4 shows how drug levels vary with time. The
concentration-time profiles over each interdose interval
look the same since, in principle, there is an
infinite number of implied doses.
Figure 6.4. Steady-state with multiple infusions. The dose is given at time t. The interdose interval is I. Steady-state levels can be predicted between times t and t+I.
Example:
TIME AMT RATE SS II
16 10. 5. 1 6
Here, infusions, each 10 units and of duration 2 (=10/5),
are assumed to have been given 6 hours apart, with the last
of these started at time 4PM, at which time steady-state is
reached. The daily dose times were 4 AM, 10 AM, 4 PM, and 10
PM. Again, the value of TIME has no effect on the computed
amounts but is important in relation to the records that
follow. Steady-state levels can be predicted between times
16 (4 PM) and 22 (10 PM) (provided there are no further
doses introduced during this interval).
These are dose events having
AMT=0, RATE>0, SS=1, and II=0. Such an event consists of
infusion with the stated rate, starting at time
 , and ending at the time on the dose event record.
Bioavailability fractions do not apply to these doses.
, and ending at the time on the dose event record.
Bioavailability fractions do not apply to these doses.
Figure 6.5 shows how drug levels vary with time.
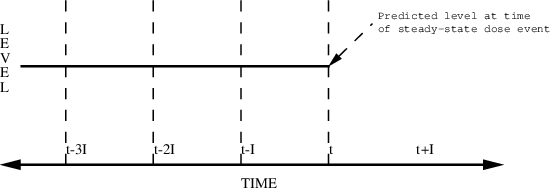
Figure 6.5. Steady-state with constant infusion. Steady-state level can be predicted only at time t.
Example:
TIME RATE SS
16 2. 1
Here, a steady-state infusion at rate 2 is specified as
ending at 4 PM. A steady-state level can be predicted only
at this time.
Doses with SS=2 are exactly like doses with SS=1. Doses with SS=2 are similar to non-steady-state doses in that compartment amounts are computed in two steps. First, compartment amounts are computed at the time on the dose event record based on the prior dosing history of the system. Second, steady-state amounts are computed from the dosing information on the record and added to the existing compartment amounts. Thus, if the kinetics are linear, this results in an application of the superposition principle wherein the amounts in the system resulting from doses described by dose event records preceding the time of the steady-state dose are superposed on the (steady-state) amounts in the system resulting from the steady-state dose and the implied doses.
As with any steady-state dose, the steady-state amounts are obtained using the values of the pharmacokinetic parameters computed from the information on the steady-state dose record. In the case that SS=2, though, if these values differ from those computed from the information on the previous dose record(s), then the compartment amounts at the time in the steady-state dose record are not truly steady-state amounts, and the computed steady-state levels are not valid predictions. PREDPP will not detect this error. We emphasize that superposition is only valid with linear kinetic systems; all the kinetic systems (ADVANs) discussed in this text are linear.
SS=2 records can be used to achieve the specification of complicated dosing regimens. For example, Figure 6.6 shows how drug levels vary with time when two different doses are alternated. In this illustration, two steady-state doses are specified, each with interdose interval I and with time between the two steady-state doses equal to I/2. Even more complex patterns are possible.
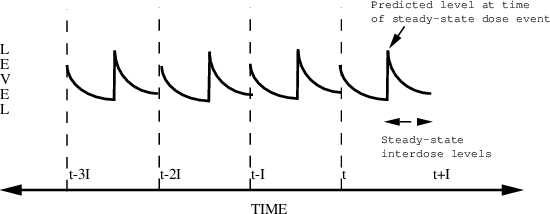
Figure 6.6. Multiple steady-state doses. Two separate steady-state doses are given. As pictured, they are each bolus doses, but they do not have to be. The first dose event record is at time t. The second dose event record is at time t+I/2. They each have interdose interval I. Steady-state levels can be predicted between times t+I/2 and and t+I.
Example:
TIME AMT SS II
8 10. 1 24
20 15. 2 24
This describes the following dosing regimen: a dose of 10
units every morning at 8 AM and a dose of 15 units every
evening at 8 PM (20 hours is 12 hours past 8). Note that
steady-state is not truly established until after the
second dose record; any observation events interposed
between the two dose records will reflect only the first
steady-state dosage (i.e., 10 units every 8 AM). Another way
to achieve the same steady-state is by the following:
Example:
TIME AMT SS II
20 10. 1 12
20 5. 2 24
This describes doses of 10 units every 12 hours, the last of
which is given at 8 PM (i.e. at 8 AM and 8 PM daily), plus
additional doses of 5 units at 8 PM daily. In both examples,
the steady-state levels can be predicted from time 20 hours
to time 32 hours.
Non-steady-state dose records
may appear before, among, or after steady-state dose
records. Such a dose record may occur before a
steady-state dose record to reflect a transient dose given
among a series of regular doses leading to steady-state, but
which is not a part of this series. E.g., a patient who has
been maintained at steady-state takes an extra dose by
mistake shortly before his appointment. A non-steady-state
dose record may occur after a steady-state dose
record in order to continue the pattern of doses beyond the
steady-state dose. Ordinarily, steady-state levels can only
be predicted between
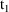 , the time on the steady-state dose record, and
, the time on the steady-state dose record, and
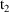 , the sum of
, the sum of
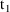 and the interdose interval. If it is not only necessary to
compute a steady-state prediction between
and the interdose interval. If it is not only necessary to
compute a steady-state prediction between
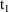 and
and
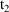 , but also after
, but also after
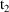 , then there must also occur one or more non-steady-state
dose records at
, then there must also occur one or more non-steady-state
dose records at
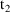 ,
,
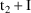 , etc. with doses just like the steady-state dose. (The
"additional doses" data item, labeled ADDL, is
especially useful for this purpose; see Chapter 12, Section
2.4.)
, etc. with doses just like the steady-state dose. (The
"additional doses" data item, labeled ADDL, is
especially useful for this purpose; see Chapter 12, Section
2.4.)
Example:
TIME AMT SS II
8 10. 1 24
20 15. 2 24
32 10. 0 0
44 15. 0 0
Here, the last two records continue the steady-state pattern
of the first two. Steady-state levels may be predicted
between times 20 and 56.
Similarly, a steady-state
constant infusion may be extended with a non-steady-state
infusion. In the example below, steady-state levels can be
predicted from time 0 to time 100.
TIME RATE AMT SS
0 30 0. 1
0 30 3000. 0
In this section we show how
urine collections and observations of urine concentration,
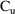 , can be described. The first-time reader may prefer to
return to this section after reading Section 4.3.3 of
Chapter 7. As an example, consider the one-compartment model
with first-order absorption (ADVAN2). The sequence of events
is:
, can be described. The first-time reader may prefer to
return to this section after reading Section 4.3.3 of
Chapter 7. As an example, consider the one-compartment model
with first-order absorption (ADVAN2). The sequence of events
is:
6:00 AM A bolus dose of 100 is given.
8:00 AM A urine collection is started.
9:30 AM
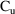 and urine volume (UVOL) are measured and a new collection is
started.
and urine volume (UVOL) are measured and a new collection is
started.
11:45 AM
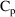 ,
,
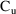 , and urine volume are measured.
, and urine volume are measured.
The $INPUT record is:
$INPUT ID TIME EVID UVOL DV CMT AMT
The data records appear as follows:
ID TIME EVID UVOL DV CMT AMT
1 6.00 1 0 0 1 100
1 8.00 2 0 0 3 0
1 9.50 0 75 .058 -3 0
1 9.50 2 0 0 3 0
1 11.75 0 100 .067 -3 0
1 11.75 0 100 5.80 2 0
Notice that urine collections start with an other type event
record (EVID=2) whose CMT contains the number of the output
compartment, the effect of which is to turn this compartment
on at 8AM, i.e. to begin accounting for the amount of drug
appearing in this compartment from 8AM. Because other type
event records are included, the EVID data item must
be present in the data. The CMT data item must be present in
all event records since it is needed to refer to the output
compartment in at least one record. Care must be taken to
use correct values for the CMT data item; default values
used when this data item is not present are not relevant in
this case. The DV value on the observation record at 9:30 is
the measured
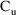 . Because the value of CMT is negative, the output
compartment is also turned off at 9:30. Since the collection
is to continue, the compartment must be explicitly turned on
again (the fourth record). Note that UVOL is recorded on
both observation records at time 11:45. Strictly speaking,
it need only be recorded on the second (
. Because the value of CMT is negative, the output
compartment is also turned off at 9:30. Since the collection
is to continue, the compartment must be explicitly turned on
again (the fourth record). Note that UVOL is recorded on
both observation records at time 11:45. Strictly speaking,
it need only be recorded on the second (
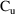 observation). This point is discussed further in Chapter 7,
Section 4.3.3. See also Chapter 12, Section 7, for a
modification to this data file for output-type
compartments.
observation). This point is discussed further in Chapter 7,
Section 4.3.3. See also Chapter 12, Section 7, for a
modification to this data file for output-type
compartments.
This section discusses in more detail the ways in which the Data Preprocessor can modify data, and discusses when a format specification should be included in the $DATA record.
Sometimes the data contains
clock times hh:mm (e.g., the time 1:30 PM is recorded as
13:30). With NONMEM 7.3, clock times may also include the
seconds hh:mm:ss. These times must be converted to
decimal-valued times (e.g., 13.5). The Data Preprocessor can
perform this task when it is processing unformatted data.
Within an individual record, the Data Preprocessor replaces
the TIME value in the first data record with 0, and then
replaces subsequent records’ TIME values with the
relative time (i.e., the number of hours elapsed since the
first record). (The TIME value is also reset to 0 on a reset
(EVID=3) or reset-dose (EVID=4) record.) Here is an example
of relative time calculation:
Contents of original file: Contents of new file:
ID TIME ID TIME
1 9:15 1 0.00
1 9:30 1 0.25
1 10 1 0.75
1 14:40 1 5.42
1 32.5 1 23.25
2 8 2 0.00
2 8.0 2 0.00
2 44:50 2 36.83
2 58 2 50.00 The presence of the colon
":" in the TIME data item of at least one record
of the data causes the Data Preprocessor to convert all the
TIME values to elapsed values. Elapsed times are also called
relative times. Note that recorded data (lines 5, 8,
and 9 of the original file) spanned more than one day. The
user had to add 24 to the TIME values on each day subsequent
to the first to communicate the correct times to the Data
Preprocessor.
Here is another way the above
data could have been recorded, using a data item called DATE
whose value is 1 for the first day, 2 for the second day,
and so on. This allows TIME values to be recorded more
naturally using values in the range 0-24.
Contents of original file: Contents of new file:
ID DATE TIME ID DATE TIME
1 1 9:15 1 1 0.00
1 1 9:30 1 1 0.25
1 1 10 1 1 0.75
1 1 14:40 1 1 5.42
1 2 8.5 1 2 23.25
2 1 8 2 1 0.00
2 1 8.0 2 1 0.00
2 2 20:50 2 2 36.83
2 3 10 2 3 50.00 The DATE data item is of
significance only to the Data Preprocessor; NONMEM-PREDPP
will not make use of it. Even if there are no ":"
characters among the TIME values, the existence of a DATE
data item will cause the Data Preprocessor to replace TIME
values by relative times.
The Date data item (DATE)
can also be used to record calendar dates in month-day-year
format. Any alphabetic character (e.g., / or -) can be used
to separate the components. Here is a third way the same
example could be recorded:
Contents of original file: Contents of new file:
ID DATE=DROP TIME ID TIME
1 10-1-86 9:15 1 0.00
1 10-1-86 9:30 1 0.25
1 10-1-86 10 1 0.75
1 10-1-86 14:40 1 5.42
1 10-2-86 8.5 1 23.25
2 10-12 8 2 0.00
2 10-12 8.0 2 0.00
2 10-13 20:50 2 36.83
2 10-14 10 2 50.00
This example illustrates two features. First, when calendar dates are used, the DATE item should be specified as "DATE=DROP", so that the data item is omitted from the new data file (see Section 5.3). Otherwise, the alphabetic characters which separate the components will cause read errors when NONMEM reads the data. Second, the year value is optional; only month and date were actually needed. (Within a single individual record, however, either all dates should specify a year, or none should.)
Data labels DAT1, DAT2, and DAT3
are also recognized by the Data Preprocessor and can be used
instead of DATE. The label given to the Date data item
describes its format:
DATE month day year
DAT1 day month year
DAT2 year month day
DAT3 year day month
If only one of the three
components is present, it is assumed to be the day†.
----------
† In this case
only, the Date data item may be zero or negative. Day
-1 means one day prior to day 0.
----------
If only two components are present, they are assumed to be month and day (with DATE and DAT2) or day and month (with DAT1 and DAT3). The year may be omitted or given as 1, 2, 3, or 4 digits.
The units of the relative TIME values resulting from the Data Preprocessor’s day-time translation are hours. If the correct units for relative time should be days, then the TRANSLATE option of the $DATA record may be used to request that hours to be converted to days. For example,
$DATA filename
TRANSLATE(TIME/24)
or
$DATA filename TRANSLATE(TIME/24.000)
With the former, values of TIME have two significant digits, e.g., xxxx.xx. With the latter, they have three significant digits, e.g., xxxx.xxx.
With NONMEM 7.3, more general conversions are possible. Any value may be given for dividing time and II values, and any precision may be requested. Examples are:
$DATA filename
TRANSLATE(TIME/1.0000)
or
$DATA filename
TRANSLATE(TIME/1/4)
for formatting times in FDATA with 4 digits to the right of
the decimal. Another example is
$DATA filename
TRANSLATE(II/0.01/6)
which divides II values by 0.01, and writes 6 digits to the
right of the decimal for the II data item. See Guide VIII
for more information.
The user may supply 4 digit years starting (e.g.) "19" and "20", and such dates are processed correctly. (Three digit years "000"-"999" are permitted, but would represent exactly those years, and should not normally be used.) If the year is omitted, it is assumed to be a non-leap year. A problem arises when the year supplied, but has only 1 or 2 digits. Such years are assumed by default to be in the 1900’s. If this is not a correct assumption, two errors may be made by the Data Preprocessor when computing relative times. First, 1900 was not a leap year, but 2000 is a leap year. Hence, if consecutive dates in a data file are 02-28-00 and 03-01-00 (signifying February 28 and March 1, 2000), an elapsed time of 24 hours, rather than 48 hours, is computed. Second, if consecutive dates have years 99 and 00, the computed elapsed time is negative and an error message is generated.
With NONMEM V and later versions there is a constant LAST20. The value of LAST20 is a 2 digit number nn that specifies the highest 2-digit year that is assumed to be in the 21st. century, i.e., to represent 20nn rather than 19nn. For example, with LAST20=50, then 1 and 2 digit years are interpreted as follows:
00-50 represents 2000-2050
51-99 represents 1951-1999
The elapsed time between 02-28-00 and 03-01-00 is calculated to be 48 hours, and the elapsed time calculated between the years 99 (1999) and 00 (2000) is positive.
There are two ways that a value for LAST20 can be specified.
First, when NM-TRAN is
installed, a value is given to constant LAST20 in
TrGlobal.f90 (in the resource directory): DATA LAST20
The default value of this constant in the distribution
medium is 50. Please ask your system support department if
they modified the LAST20 constant when NM-TRAN was
installed.
Regardless of what value was assigned to the LAST20 constant in TrGlobal.f90, there is an option LAST20 of the $DATA record that may be used to specify the value of the constant for the current run. For example:
$DATA filename LAST20=50
This insures that all 1 and 2 digit years are interpreted as above (2000-2050; 1951-1999).
There may be two circumstances such that 1 or 2 digit years are recorded as 00, 01, ... (equivalently, 0, 1, ...). First, these may represent the years 2000, 2001, etc. Or, they may represent years 0, 1, etc., of a study. Suppose the latter is the case, and that none of the years of the study was a leap year. Then if LAST20 is set to a value greater than -1, the year 0 is assumed incorrectly to be the leap year 2000, and elapsed times may be computed incorrectly. The Data Preprocessor issues a warning message under the following circumstances:
|
1) |
The year is recorded as "00" or "0", | |
|
2) |
The value of LAST20 is greater than -1 by default (so that the year is understood to be 2000), and | |
|
3) |
The LAST20 option of the $DATA record was not used to modify LAST20 for this run. |
The warning message is as follows:
(DATA WARNING 3) RECORD 3, DATA ITEM 3: 01-01-00 THE YEAR IS ASSUMED TO BE 2000 (A LEAP YEAR). IF THIS IS INCORRECT, USE $DATA’S LAST20 OPTION TO OVERRIDE THE DEFAULT VALUE OF LAST20 IN NMTRAN’S ABLOCK, OR CHANGE THE DEFAULT: 50
Suppose these warning messages are appropriate, that is, year "00" (or "0") should not be a leap year. The LAST20 option of the $DATA record may be used to specify that such years are in the 1900’s for the current data set:
$DATA filename LAST20=-1
A constant LYWARN is defined in NM-TRAN’s ABLOCK module. The default value of LYWARN is 1 ("data warning message 3 enabled"). If the value of LYWARN is set to 0 ("data warning message 3 disabled") and NM-TRAN is recompiled, then the warning message is suppressed for all runs.
When the input data is unformatted and PREDPP is being used, the Interdose Interval (II) data item is checked for values containing a colon (:). Any such value is assumed to signal a clock time hh:mm. The minutes portion is converted to a decimal number containing as many decimal places as digits in the original. E.g., the value ":30" is replaced by ".50". This conversion is performed whether or not day-time translation is also being done.
When the data is from a single
individual, the Data Preprocessor will almost always
generate an ID data item‡.
----------
‡ Section 4.2 of
Chapter 12 discusses the L1 data item, which is used to
prevent NM-TRAN from generating an Identification data item
for individual data.
----------
It does this whether or not PREDPP is used. This is done because, when the data is from a single individual, the ID data item must take on very special non-constant values for NONMEM. The generated ID data item is given the label ".ID." (i.e., ID surrounded by dots). If this data item is to be shown in any NONMEM output (e.g., in a table), it must be referred to on subsequent NM-TRAN records by this label.
When PREDPP is used, the Data Preprocessor will always generate the required EVID data item if it is not already listed on the $INPUT record. (This was discussed in Section 7.3 above.)
When PREDPP is used, the Data Preprocessor will always generate the MDV (Missing Dependent Variable) data item if it is not already listed on the $INPUT record.
These data items are generated by the Data Preprocessor whether or not a format specification was coded on the $INPUT record. They are appended to the end of each data record.
When coding the $DATA record, you will need to decide whether to include a FORTRAN format specification describing the data file or to omit it and let the Data Preprocessor construct it. Here are some guidelines in making this decision.
A format specification is required when:
Some data values are left blank on some data records, without having the value 0 or . (or a pair of commas) to hold the place of the missing value.
Some data values are adjacent on some data records; they are not separated by a space or a comma.
The data records span multiple physical records; that is, the character / is needed in the format specification. (The Data Preprocessor may generate such a format specification for the NONMEM data set; we are speaking here of the NM-TRAN data set.) The NOOPEN option of $DATA is used.
A format specification should not be present when:
The $INPUT record includes DROP as a data item label or synonym.
Day-time translation is desired.
II conversion is desired.
Commas are used to separate the data items.
The data values are not lined up into columns with uniform width, so that no format specification can be written to describe the file.
The IGNORE/ACCEPT option of $DATA is used to drop records from the data set.
Many data files do not fall in either category. A format specification is optional for such files.
NM-TRAN performs more checks on the data file when there is no format specification. Some features of NM-TRAN are the same with or without a format specification.
Comment records may be used.
NM-TRAN appends EVID, MDV, .ID., as needed.
NM-TRAN checks for blank records, and the BLANKOK option of
$DATA may be used.
NM-TRAN gives a warning for unusual characters in the data
file.
NM-TRAN counts records and supplies the count in FCON.
It is always possible to omit (skip) data items that are not of interest for a given NONMEM run. When a format specification is coded, two things must be done: first, replace the data item’s specification by an "X" specification (e.g., replace F8.0 by 8X) and second, delete the data item’s label from the $INPUT record. When no format specification is coded, all that need be done is to replace the data item’s label in the $INPUT record by DROP (or include DROP as a synonym).